The role of alternately spliced forms of PKR in osteosarcoma progression and metastases.
Osteosarcoma (OS) is an insidious tumor typically affecting the long-bones bones of the arms and legs. It strikes mostly adolescents and young adults during the major growth phase in life with an average age of diagnosis being 15 years of age. While the primary tumor is treatable with surgery and chemotherapy, with a 70% survival rate in early diagnosed patients, it is the frequent metastases of this tumor to the lungs that most often prove fatal. About 30% of osteosarcoma patients present with metastases upon initial diagnosis, and of those that don’t, approximately 90% will develop metastases within 6 months to 3 years (Schwab, et al., 2012; Rivera-Valentin, et al., 2015). Studies into the molecular mechanisms involved in OS development and progression have implicated perturbations in multiple signal transduction pathways regulating apoptosis, the cell cycle, cell growth and translation. Only within the last 5 years have studies begun to assess the role of inflammatory/stress signaling in OS. Normal bone growth in childhood and adolescents is a result of both growth promoting factors (hormones, growth factors/cytokines) and physical stresses to the interested tissues that are then translated into a biological stress response. Interestingly, a number of key players in this stress response are shared with other inflammatory/stress responses (Yuan, et al., 2017).
The double-strand RNA dependent kinase, PKR, is a sentinel innate immune inflammatory/stress activated kinase that plays a central role in the cellular response to viral infection, interferon and cytotoxic cytokines, Toll-like receptor activation, DNA damage and nuclear stress, and oxidative damage (Garcia-Ortega et al., 2017). Recent immunohistochemical data from our group has shown that PKR expression is elevated in approximately 80% of osteosarcoma patient samples, as compared to control bone tissue, with active PKR (p-T451 PKR) staining being found near border regions of the tumor vasculature primarily in those samples expressing the greatest amounts of PKR (Fig. 1).
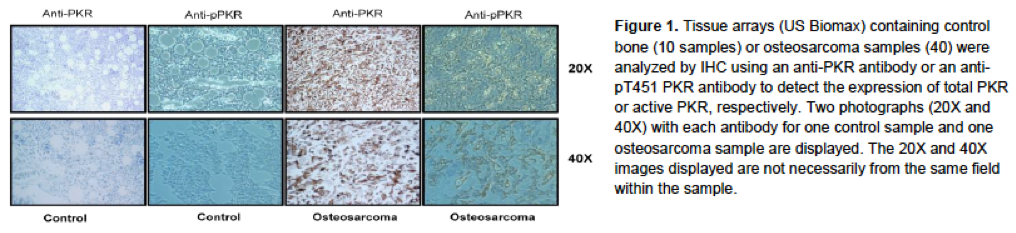 |
Analysis of PKR mRNA and protein expression in four OS cell lines, U2OS, SaOS-2, HOS and MG-63, and the control osteoblastic cell line hFOB1.19 indicated that each of the cell lines expressed PKR. Interestingly, OS cell lines produced a significant amount of alternatively spliced PKR mRNAs (57%, 61%, 75% and 50%, respectively, compared to 22% in hFOB1.19), many which have not been previously documented. Added to this, PKR overexpression in two OS cell lines, U2OS and SaOS-2, was found to enhance attachment-independent growth and migration (Piazzi et al., 2019).
By defining which PKR isoforms are promoters of OS and which are not and identifying the role of tumor promoting isoforms in OS development and progression, we may begin to understand the relationship between stress/inflammatory signaling and OS. Moreover, PKR represents an excellent target for the design of inhibiting peptides, antibodies or small molecule inhibitors to disrupt interactions/activity critical for tumorigenesis.
Our Lab is trying to answer the following question:
- Which alternatively spliced PKR transcript are present in OS patient samples?
- What cellular proteins interact with each PKR isoform?
- Do the PKR isoforms influence OS progression?
- Do the PKR isoforms influence the response of OS to chemotherapeutic treatment?
Bibliography:
Blalock,WL., Piazzi,M., Bavelloni,A., et al. Identification of the PKR Nuclear Interactome Reveals Roles in Ribosome Biogenesis, mRNA Processing and Cell Division. (2014). J Cell Physiol. 229: 1047-1060.
Garcia-Ortega,MB., Lopez,GJ., Jimenez,G., et al. Clinical and therapeutic potential of protein kinase PKR in cancer and metabolism. (2017). Expert Rev Mol Med.19:e9.
Piazzi,M., Bavelloni,A., Greco,S., et al. Expression of the double-stranded RNA-dependent kinase PKR influences osteosarcoma attachment independent growth, migration, and invasion. (2020) J Cell Physiol. 235:1103-1119.
Rivera-Valentin,RK., Zhu,L., Hughes,DP. Bone Sarcomas in Pediatrics: Progress in Our Understanding of Tumor Biology and Implications for Therapy. (2015). Paediatr Drugs. 17:257-271.
Schwab,JH., Springfield,DS., Raskin,KA., et al. What’s new in primary bone tumors. (2012). J Bone Joint Surg Am. 94:1913-1919.
Yuan,Y., Zhang,L., Tong,X., et al. Mechanical Stress Regulates Bone Metabolism Through MicroRNAs. (2017). J Cell Physiol. 232:1239-1245
