PRIN2017: Epigenetic Regulation of Nuclear Inositides in Bone Marrow Microenvironment and MDS/AML Progression: New Targets, Therapy and Drugs
Aberrant nuclear inositide signalling negatively influences hematopoietic cell proliferation and apoptosis. Indeed, the activation of the PI3K/Akt/mTOR axis is associated with leukemic progression, and the epigenetic deregulation of Phosphoinositide-Phospholipase C (PI-PLC) beta1 is involved in the pathogenesis of hematological malignancies.
Myelodysplastic Syndromes (MDS) are a heterogeneous group of clonal diseases affecting the hematopoietic stem cells that are characterized by an increased although variable risk of evolution in Acute Myeloid Leukemia (AML) and a gene promoter hypermethylation. Moreover, the acquisition of epigenetic alterations negatively influences the MDS hematopoietic stem cells, perturbing their unstable bone marrow niche and promoting the leukemic progression. That is why MDS patients are currently treated with two cytosine analogues having a demethylating effect. Azacitidine, in particular, can clinically improve MDS patients’ survival, quality of life and delay AML evolution. Moreover, it is also used in AML patients who are not candidates to intensive treatments or are refractory to other drugs, becoming a valuable alternative also in these subjects. However, azacitidine has to be continuously administered, until a clinical response is detected, and the lack of reliable predictive molecular markers of therapy response is still an important issue, above all for patients showing an unexpected loss of response during treatment, as these subjects are often refractory to other treatments.
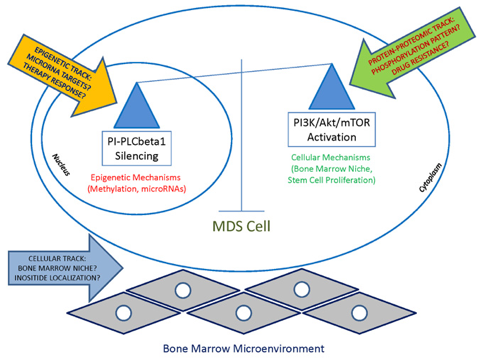
Figure 1. Graphical outline of the Research project
At a molecular level, azacitidine induces promoter demethylation of critical genes, it regulates the expression of microRNAs and favors the restoration of a correct myeloid differentiation, targeting PI-PLCbeta1 among many other genes.
Our research activity aims at studying the epigenetic processes underlying the MDS leukemic progression, focusing not only on microRNA signatures, but also identifying specific microRNA-inositides interactions that could become druggable targets (Figure 1).
COLLABORATIONS
University of Bologna
University of Modena and Reggio Emilia
Department of Experimental Medicine of the University of Bologna
University of Oxford, UK
