DDX11 loss causes replication stress and pharmacologically exploitable DNA repair defects
Mutations in BRCA1 and BRCA2 tumor suppressor genes predispose individuals to breast and ovarian cancers. These cancers are treated in the clinic with platinum drugs and inhibitors of polyADP-ribose polymerase (PARP), which cause lesions requiring homologous recombination repair activities mediated by BRCA1 and BRCA2. However, BRCA1 and BRCA2 tumors often acquire resistance to chemotherapy, either by somatic reversion or by acquiring homologous recombination proficiency, leading to aggressive tumor growth.
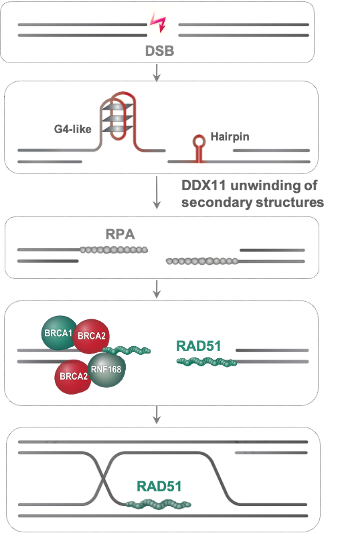 Thus, there is an urgent need to identify ways to potentiate the therapy and overcome acquired resistance of homologous recombination-deficient cancer cells. In a recent work, published in Proceedings of the National Academy of Science USA (PNAS) by researchers of IGM and IFOM, Dana Branzei and Nanda Kumar Jegadesan identify that the helicase DDX11 is critical to mitigate replication stress in a range of cancers by facilitating upstream steps in homologous recombination repair related to efficient resection of double strand breaks. Importantly, DDX11 acts in manners non-redundant with BRCA1 and BRCA2. Strikingly, this study shows thar targeting DDX11 improves chemotherapy response in both chemotherapy sensitive and drug-resistant BRCA1/2-mutated cancers that regained homologous recombination proficiency by suppressor mutation or somatic reversion. Thus, DDX11 provides a mechanism of replication stress tolerance in cancers, a finding that can be exploited therapeutically in the future through the development of specific inhibitors of DDX11 helicase activity.
Thus, there is an urgent need to identify ways to potentiate the therapy and overcome acquired resistance of homologous recombination-deficient cancer cells. In a recent work, published in Proceedings of the National Academy of Science USA (PNAS) by researchers of IGM and IFOM, Dana Branzei and Nanda Kumar Jegadesan identify that the helicase DDX11 is critical to mitigate replication stress in a range of cancers by facilitating upstream steps in homologous recombination repair related to efficient resection of double strand breaks. Importantly, DDX11 acts in manners non-redundant with BRCA1 and BRCA2. Strikingly, this study shows thar targeting DDX11 improves chemotherapy response in both chemotherapy sensitive and drug-resistant BRCA1/2-mutated cancers that regained homologous recombination proficiency by suppressor mutation or somatic reversion. Thus, DDX11 provides a mechanism of replication stress tolerance in cancers, a finding that can be exploited therapeutically in the future through the development of specific inhibitors of DDX11 helicase activity.
Nanda Kumar Jegadesan and Dana Branzei. DDX11 loss causes replication stress and pharmacologically exploitable DNA repair defects. Proc Natl Acad Sci, doi:10.1073/pnas.2024258118.
